Ayush.gov.in recives any estimated n/a unique visitors and n/a unique page views per day. Revenue gained from these much visits may be n/a per day from various advertising sources. The estimated worth of site is n/a.
- Website Age
n/a
- Alexa Rank no-data
- Country
 India
India
- IP Address
164.100.166.73
google ads
HTML SIZE INFORMATION
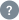
Text / Code Ratio
15.01 %
ayush.gov.in has a website text/code ratio of 15.01 %. Search engine crawlers tend to not pick up pages with inadequate content.
IMPORTANT HTML TAGS AND COUNTS

H2
| No |
Text |
| 1 |
Covıd - 19 related ınformation |
| 2 |
Awareness |
H5
| No |
Text |
| 1 |
© 2020 ministry of ayush. government of ındia, all rights reserved. |
Text Styling

- STRONG6
- B0
- EM0
- I0
- U0
- CITE0
STRONG
| No |
Text |
| 1 |
Dashboard AYUSH for Covid - 19 |
| 2 |
Helpline Number : |
| 3 |
Toll Free : |
| 4 |
Helpline Email ID : |
| 5 |
States & Union Territories (View pdf) |
| 6 |
Arogya Setu App |
LINK ANALYSIS
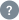
Total Link Count: 29
Internal Link Count
: 24 
| No |
Text |
Type |
| 1 |
आयुष मंत्रालय Ministry of AYUSH |
image |
| 2 |
Home |
text |
| 3 |
Awareness |
text |
| 4 |
- |
image |
| 5 |
ncov2019@gov.in |
text |
| 6 |
(a) NATUROPATHY Guidelines |
text |
| 7 |
(b) SIDDHA Guidelines |
text |
| 8 |
(c) HOMOEOPATHIC Guidelines |
text |
| 9 |
(d) UNANI Guidelines |
text |
| 10 |
(e) AYURVEDA Guidelines |
text |
| 11 |
(f) YOGA Guidelines |
text |
| 12 |
Clinical Protocol Guideline |
text |
| 13 |
(a) Guidelines of Clinical Evaluation |
text |
| 14 |
(b) Guidelines of Drug Development |
text |
| 15 |
(c) Guidelines of Safety Toxicity |
text |
| 16 |
Notification for undertaking research on Covid 19 through Ayurveda, Unani, Siddha and Homoeopathy systems |
text |
| 17 |
Rejoinder issued to the New York Times for correct representation of facts about Government of India's approach to adopting AYUSH solutions during the Covid- 19 crisis |
text |
| 18 |
ADVISORY FROM MINISTRY OF AYUSH FOR MEETING THE CHALLENGE ARISING OUT OF SPREAD OF CORONA VIRUS (COVID-19) IN INDIA |
text |
| 19 |
Telemedicine Practices Guidelines by CCIM |
text |
| 20 |
Telemedicine Practice Guidelines by CCH |
text |
| 21 |
Training resources for Covid 19 Management |
text |
| 22 |
Ayurveda’s immunity boosting measures for self care during COVID 19 crisis |
text |
| 23 |
Order to stop and prevent publicity and advertis*****t of AYUSH-related claims for COVID-19 treatment |
text |
| 24 |
Expediting the process for grant of approval-license-renewal of license for manufacturing of ASU immunity boosting healthcare products and sanitizers |
text |
External Link Count
: 5 
| No |
Text |
Type |
| 1 |
Dashboard AYUSH for Covid - 19 |
text |
| 2 |
Main AYUSH Website |
text |
| 3 |
States & Union Territories (View pdf) |
text |
| 4 |
- |
image |
| 5 |
- |
image |
Nofollow Link Count
: 0
Title Link Count
: 0
WEBSITE SERVER INFORMATION
- Service Provider (ISP)
- National Informatics Centre
- Hosted IP Address
- 164.100.166.73
- Hosted Country
 India
India- Host Region
- Tamil Nadu , Chennai
- Latitude and Longitude
- 13.086 : 80.2751
WEBSITES USING THE SAME C CLASS IP


 India
India


 External Link Count
: 5
External Link Count
: 5 
 164.100.166.50
164.100.166.50 164.100.166.53
164.100.166.53